This week in PLOS Biology
In PLOS Biology this week, you can read about how plants cope with arsenic in the soil, the lengthening of tubular biological structures, disrupting protein translocation, and the need for transparency in grant reviews.
Living with Arsenic

Arsenic is nasty stuff, being toxic even at very low levels to most living organisms, and a hazardous environmental carcinogen for some human populations. Its abundance in some soils means that it can get into plants, where it runs the risk of contaminating human foodstuffs. Being able to control the levels of arsenic in food crops is therefore of some importance, and this involves knowing how plants have evolved to deal with the element. A genome-wide association study allowed Dai-Yin Chao, David Salt and colleagues to exploit natural variations in arsenic accumulation in wild Arabidopsis thaliana isolates. This identified the enzyme in plants that transforms arsenate into arsenite, as arsenate reductase HAC1. Formation of arsenite allows its extrusion into the soil and thereby controlling accumulation of the toxin – in the absence of HAC1, levels of arsenic in the plant rises 300-fold. Read more in the Synopsis.
Growing a Longer Tube
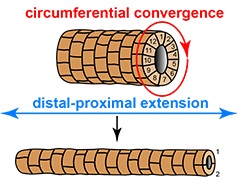
As animal embryos develop, many organs require the formation of tubular structures by orchestrated movements of cells. A new study by Aditya Saxena, Barry Denholm, Helen Skaer and colleagues, focuses on one such instance – the formation of renal tubules (“Malpighian tubules“) in the fruit fly. These originate as little buds on the hindgut, but then lengthen dramatically within a few hours. The authors show here how graded signalling by the epidermal growth factor Spitz provides the cells with axial information for polarized myosin pulses. These contractions shorten the cells in their circumferential dimension, driving them to intercalate and thereby causing elongation of the tubule.
Inhibiting Protein Translocation – Specifically
There are many ways in which one could in principle manipulate levels of a given protein. Kurt Vermeire, Kai-Uwe Kalies, Mark Marsh and colleagues study the small anti-HIV drug CADA, and find that it targets the membrane translocation of the precursor of the crucial T-cell protein CD4. They show that it does so in a novel fashion, by specifically binding to pre-CD4’s signal peptide during co-translational translocation (the step where the ribosome shoves the nascent protein through the protein-conducting Sec61 translocon in the endoplasmic reticulum membrane). This locks the signal peptide in the translocon, so instead of becoming embedded in the membrane and being routed to the cell surface, the CD4 precursor is diverted to the cytosolic degradation machinery. The effect seems to be specific to this protein and raises the possibility that other membrane proteins could be similarly targeted.
Grant Application Review: The Case for Transparency
How much do we know about the process by which the relative merits of research proposals are assessed? David Gurwitz, Elena Milanesi and Thomas Koenig propose that public funding agencies should be more transparent in awarding research grants to allow researchers and the public better insight into decision making.
