Understanding Images: Seeing Myopia for What it Is- Potentially Treatable
In a post reflecting on August’s PLOS Genetics issue image, Andrei Tkatchenko explains the science behind the image.
Author: Andrei V. Tkatchenko, Columbia University Medical Center, New York, USA
Competing interests: Andrei V. Tkatchenko is an author of the article discussed in this blog

Myopia (commonly known as nearsightedness) is the most common ocular disorder worldwide. Genetic factors are believed to play a key role in determining the impact of environmental factors such as reading and ‘nearwork’ on refractive eye development and myopia incidence; however until now, we have lacked experimental proof of this gene-environment interaction. In this issue of PLOS Genetics, using a “systems genetics” approach, which combined gene expression profiling in a monkey model of myopia, GWA studies in a human population and validation of candidate genes in a gene-targeted mouse model of myopia our study identified APLP2 as one of the genes responsible for the gene-environment interaction in myopia.
We are Facing an Epidemic of Myopia
The prevalence of myopia has increased from 25% to 44% of the adult population in the United States of America in the last 30 years, and reached more than 80% in some parts of Asia. Epidemiological data suggest that even low-grade common myopia represents a major risk factor for a number of serious ocular conditions such as cataract, glaucoma, retinal detachment, and myopic maculopathy, and represents the seventh leading cause of blindness. The increasing prevalence of myopia costs the U.S.A. nearly $7.2 billion a year for refractive correction alone, and can have negative effects on self-perception, job and activity choices. It is estimated that 2.5 billion people (1/3 of the world’s population) will be affected by myopia by 2020.
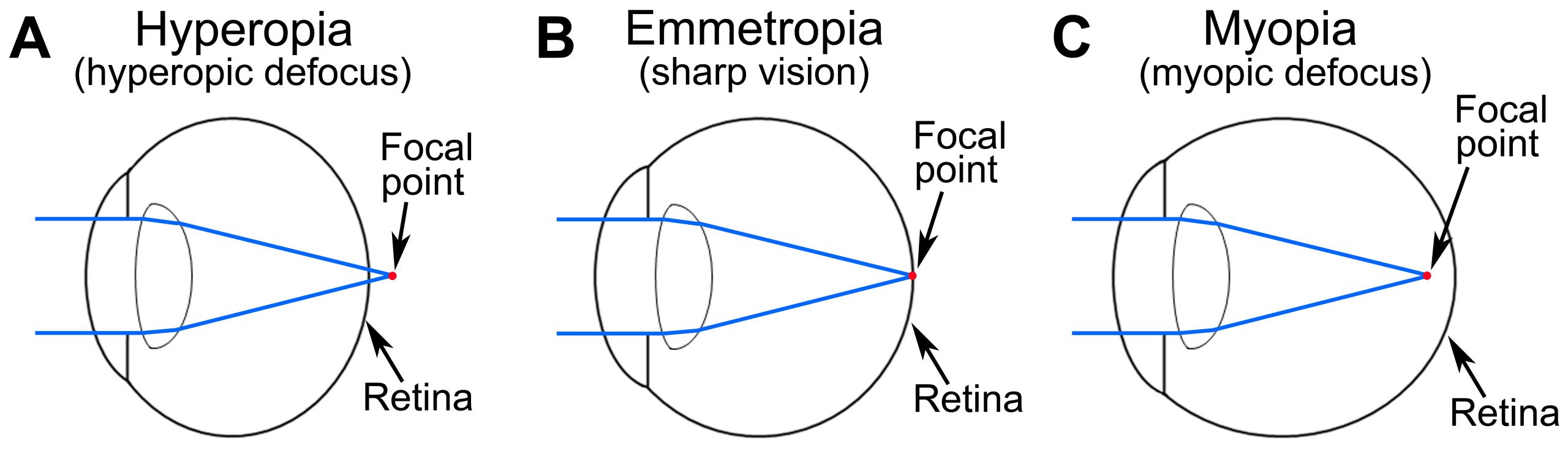
Genes Versus Environment

Human population studies suggest that environmental factors, such as nearwork and reading, play an important role in the development of myopia [1-4]. These factors are associated with a so-called “lag of accommodation”, i.e., insufficiently strong accommodative response for near objects, which places the plane of focus behind the retina when nearwork tasks are performed. The optical blur produced by the lag of accommodation is believed to be the signal that drives excessive eye growth and causes myopia [2, 5-9]. Animal studies also demonstrated that excessive eye growth and myopia can be induced by either optical blur (recapitulated in animal models by placing a diffuser in front of the eye) or hyperopic defocus (mimicked by placing a negative lens in front of the eye). Environmental factors play a very important role in the development of myopia and are responsible for the recent sharp increase in the prevalence of myopia worldwide, however the contribution of genetic factors has been estimated to be between 60% and 90%. Genetic factors play a key role in determining the impact of environmental factors, such as reading and nearwork, on refractive eye development and development of myopia. In the context of refractive eye development, understanding how genes and environmental factors interact is key to understanding the mechanisms of myopia.
A Risk Variant of APLP2 Causes Myopia in Children who Engage in Above-Average Levels of Reading
Our study found that APLP2 is associated with myopia in children and adults. Moreover, further analysis revealed that children who carry a specific version (risk variant) of APLP2 are 5 times more likely to develop myopia if they read more than 1 hour a day, thus providing evidence of interaction between APLP2 and visual environment. Functional analysis of APLP2 in the mouse model of myopia, in which APLP2 was genetically removed from the genome, confirmed gene-environment interaction between APLP2 and visual environment and demonstrated that lack of APLP2 expression leads to a dose-dependent reduction in susceptibility to myopia in mice. Lack of APLP2 also affected electrophysiological properties of the retina resulting in reduced contrast sensitivity, which correlated with reduced susceptibility to myopia. We also found that APLP2 is expressed in a specific subset of amacrine cells, which were shown to be involved in contrast processing. APLP2 expression in these cells seems to be responsible for its effect on contrast sensitivity and susceptibility to myopia.

Myopia Therapy in Sight?
With the exception of glasses, lenses and laser surgery, there are currently no treatment options for myopia. Our study provides hope that myopia can be treated. Our mouse data suggest that reducing the level of APLP2 expression in the retina can significantly reduce susceptibility to myopia. This effect seems to be achieved via modulation of the amacrine cell function, which in turn affects contrast sensitivity. Considering that reduction in APLP2 levels did not affect visual acuity, control of APLP2 expression in the retina may provide the framework for the development of pharmacological treatment options for myopia.
Further information:
- Parssinen O, Lyyra AL (1993) Myopia and myopic progression among schoolchildren: a three-year follow-up study. Invest Ophthalmol Vis Sci 34: 2794-2802.
- Goss DA (2000) Nearwork and myopia. Lancet 356: 1456-1457.
- Hepsen IF, Evereklioglu C, Bayramlar H (2001) The effect of reading and near-work on the development of myopia in emmetropic boys: a prospective, controlled, three-year follow-up study. Vision Res 41: 2511-2520.
- Saw SM, Chua WH, Hong CY, Wu HM, Chan WY, et al. (2002) Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci 43: 332-339.
- Gwiazda J, Thorn F, Bauer J, Held R (1993) Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci 34: 690-694.
- Gwiazda J, Bauer J, Thorn F, Held R (1995) A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Res 35: 1299-1304.
- Abbott ML, Schmid KL, Strang NC (1998) Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiol Opt 18: 13-20.
- Charman WN (1999) Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt 19: 126-133.
- Gwiazda JE, Hyman L, Norton TT, Hussein ME, Marsh-Tootle W, et al. (2004) Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci 45: 2143-2151.
- Tkatchenko AV, Tkatchenko TV, Guggenheim JA, Verhoeven VJM, Hysi PG, Wojciechowski R, et al. (2015) APLP2 Regulates Refractive Error and Myopia Development in Mice and Humans. PLoS Genet 11(8): e1005432. doi:10.1371/journal.pgen.1005432

This is truly exciting information, thank you for posting. I think the article is remiss though in that orthokeratology is not mentioned. While orthokeratology may not be a “cure” for myopia, for now it is one of the most effective means of decreasing myopia progression while providing clear vision throughout the day. By providing clear vision and focus at the fovea and reducing hyperopic defocus in the periphery, orthokeratology creates the optimum image shell on the retina.
Respectfully,
Paul Levine, OD, FIAO, FAAO