Understanding Images: Chimeric sugar transporters bring Greek mythology to the brewing industry
Author: EmilyClare Baker is a postdoctoral scholar at the Institute of Ecology & Evolution, University of Oregon-Eugene.
Competing Interests: EmilyClare Baker is an author of the article discussed in this blog. The authors of the discussed article, together with the Wisconsin Alumni Research Foundation, have filed a provisional patent application entitled, “POLYPEPTIDE AND YEAST CELL COMPOSITIONS AND METHODS OF USING THE SAME.” All strains and constructs are freely available for non-commercial research.
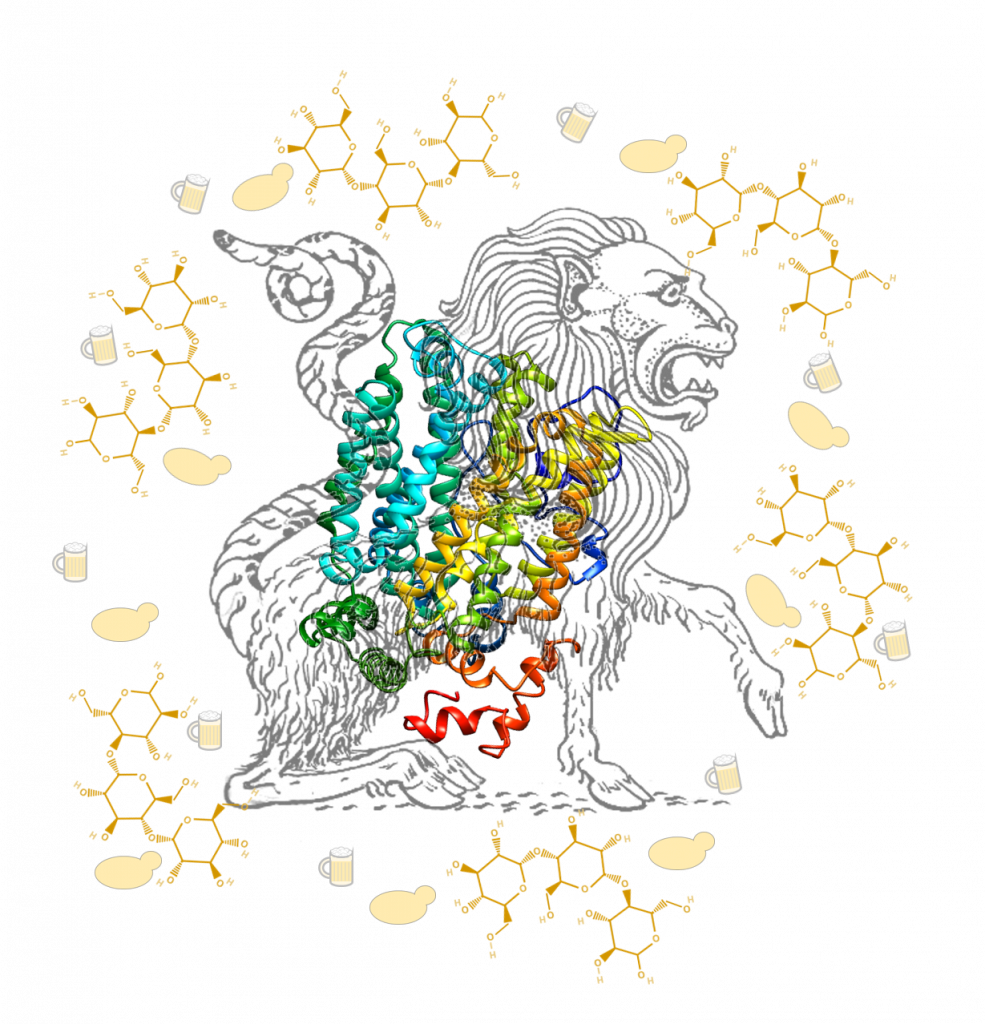
Image Caption: In Greek mythology, the chimera combined the physical characteristics of several different creatures to make a wholly new beast with attributes both like and unlike its parents. A recombination event between two maltose sugar transporter genes from the yeast Saccharomyces eubayanus resulted in a chimeric transporter that gained the ability to carry larger maltotriose sugar molecules. The uptake of maltotriose by S. eubayanus is a critical step in being able to ferment a sugar whose consumption is important for the production of high-quality beer.
Image Credit: Image of chimera has been released into the public domain by its author, Pearson Scott Foresman. Graphic created by Emily Baker.
Acknowledgements: I thank Chris Hittinger for his comments on the draft of this blog.
Understanding how domesticated organisms evolve to the specific and often extreme conditions of industrial environments can lead not only to improvements in industrial organisms, but also to insights into more general biological questions. The April 2019 issue image for PLOS Genetics shows the predicted structure of the novel chimeric protein described in Baker and Hittinger [1] surrounded by yeast, the experimental basis of our work, and mugs of beer, its industrial output. Like the chimera of Greek myth, our work and the work of the Daran lab [2] joined together elements that are usually separated. Independently, our groups combined experiments of both basic scientific and industrial interest and both resulted in cobbled-together proteins with novel functions. As with many scientific endeavors, our projects started with beer.
The characteristic clarity, flavor and low fermentation temperature of lager-style beers come from the unique yeast used in the brewing process. This yeast is a hybrid between the industrious and ubiquitous Saccharomyces cerevisiae and its distant, more reclusive relative, Saccharomyces eubayanus. In these hybrid strains, S. eubayanus contributes the preference for low temperatures, while S. cerevisiae contributes much of the alcohol production.
Given the contribution of S. eubayanus to lager brewing, a multibillion-dollar industry, there is considerable interest in developing this species as a brewing strain in its own right. A major roadblock, however, is that S. eubayanus cannot consume maltotriose. Maltotriose, a bulky trimer of glucose molecules, is a major brewing sugar and its fermentation is important for producing high quality beer. To determine if and how S. eubayanus could evolve the ability to use maltotriose, we took an experimental evolution approach. Starting with three genetically distinct strains of S. eubayanus, with three replicate populations each, we provided each replicate with an overabundance of maltotriose and a small amount of glucose to support a limited amount of growth.
After months of sluggish growth, a single replicate began growing rapidly. After confirming that increased growth was due to a newly acquired ability to consume maltotriose, we used whole-genome sequencing to pinpoint the genetic basis. We determined that maltotriose consumption was conferred by a single sugar transporter gene. Specifically, a partial gene conversion had replaced the DNA encoding about 80 amino acids in the gene MALT4 with the equivalent region from the related gene, MALT3. We named this new chimeric gene MALT434 for the ancestry pattern of the sequence. MALT4, MALT3, and MALT434 are all predicted to have very similar protein structures, but overexpression of MALT434 conferred growth on maltotriose, while MALT4 and MALT3 did not. However similar their structures and functions in their native backgrounds, it was the novel environment of MALT4 that allowed the MALT3 residues to impart the ability to transport maltotriose.
Unbeknownst to us, half a world away, the Daran Lab had embarked on a similar evolution experiment. While we relied on the native mutation rate to provide variation for natural selection, they used UV radiation to induce mutations in S. eubayanus and speed up evolution. Remarkably, they too linked maltotriose utilization in evolved strains to a chimeric transporter. The similarities between the outcomes of our independent experiments is striking. Both evolved chimeric transporters at the MALT4 locus and swapped in part of the C-terminus with MALT3 sequence (the chimera described by Brouwers et al. [2] also included a contribution from the related protein Malt1). And while parents and chimeras were all predicted to have similar structures, only overexpression of chimeras allowed growth on maltotriose.
Implications of findings
Chimeric proteins are typically associated with modularity and the recombination of independent functional units. Malt proteins lack this sort of modularity. Our work suggests that recombination within proteins outside of modular functional domains is a common mechanism to evolve novel functions through intraprotein epistasis. Indeed, the use of directed recombination between related proteins followed by selection has been used with great success in protein engineering for many years.
The striking similarity in the results between our independent experiments addresses another more fundamental question in evolutionary biology. Are the evolutionary outcomes we observe inevitable or if we were to “replay the tape of life” could different solutions arise? In the case of maltotriose transporters in Saccharomyces yeasts, our work suggests there are a limited number of evolutionary solutions available to transport this bulky saccharide. It may be that similar evolution of transporters to carry larger molecules would also involve a similar mechanism. In fact, evidence presented by Brouwers et al. [2] suggests that one of the few natively occurring maltotriose transporters in S. cerevisiae may have arisen by a similar recombination event.
Of much more general interest, these experiments have produced strains of S. eubayanus capable of consuming an important brewing sugar, opening the way for greater utilization of S. eubayanus in the brewing industry. Cheers!
References
- Baker EP, Hittinger CT (2019) Evolution of a novel chimeric maltotriose transporter in Saccharomyces eubayanus from parent proteins unable to perform this function. PLOS Genetics 15(4): e1007786. https://doi.org/10.1371/journal.pgen.1007786
- Brouwers N, Gorter de Vries AR, van den Broek M, Weening SM, Elink Schuurman TD, et al. (2019) In vivo recombination of Saccharomyces eubayanus maltose-transporter genes yields a chimeric transporter that enables maltotriose fermentation. PLOS Genetics 15(4): e1007853. https://doi.org/10.1371/journal.pgen.1007853
