This week in PLOS Biology
In PLOS Biology this week you can read about new ways to approach preclinical trials, signaling in the vertebrate retina, how Leishmania adapts to its environment and a protein which can stop bacterial protein synthesis when nutrients are low.
In their Perspective this week, Jonathan Kimmelman, Jeffrey Mogil and Ulrich Dirnagl discuss the recent consternation over the way in which preclinical investigations of new drugs are performed and reported. They argue that we first need to distinguish between ‘exploratory’ investigation – generating robust pathophysiological theories of disease – and ‘translational’ or ‘confirmatory’ investigation, which seeks to demonstrate reproducible effects in animal models. Kimmelman and colleagues say that each type of research requires different study designs and suffers from different validity threats, and that this should be taken into account in research policy.
In the vertebrate retina, an interaction between the horizontal cells and photoreceptor cells allows us to remove redundant visual information in space and time, in order optimally see a scene. But how, physiologically, does this work? Rozan Vroman, Maarten Kamermans and colleagues measured current within goldfish cone-horizontal cell synapses to answer this question. They found that the horizontal cells feed back to photoreceptors via both a very fast mechanism and by a relatively slow mechanism. This slow mechanism requires ATP release from the tips of horizontal cell dendrites, followed by hydrolysis of ATP to products that acidify the synaptic cleft. Read more in the accompanying synopsis.
A research article by Jean-Michel Ubeda, Marc Ouellette and colleagues finds that the human parasite Leishmania uses gene rearrangements and repeat-mediated amplification on a genome-wide scale as a strategy to adapt to a changing environment. This means that upon selection with either drugs or culture conditions, a subpopulation can emerge where the amplicon copy number per cell increases and this clone of cells can then expand to dominate the population.
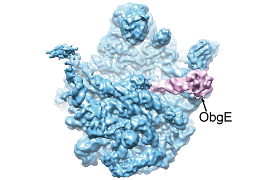
Typical bacterial cells contain tens of thousands of ribosomes, which make the proteins needed for life. However, during hard times, when nutrients (and therefore amino acids) are low, the cell needs to slam on the brakes. In their new paper, Boya Feng, Ning Gao and colleagues found that a protein called ObgE can bind to the large subunit of the ribosome, disrupting its association with the small subunit and stopping translation. They suggest that the presence of (p)ppGpp – a chemical made by bacteria in response to low levels of amino acids – causes ObgE to linger on the large subunit longer than it would in its normal role. Read more in the accompanying synopsis.