The XV Collection: A Spotlight on Spottiness
By Sally Lowell
Let’s start with a remarkable fact. Cells can, under the right conditions, organise themselves into patterns without any outside instruction. Indeed, it is the ability of cells to self-organise that makes multicellular life possible. Contemplating this, it soon becomes apparent that the only proper course of action is to become a developmental biologist and devote one’s life to trying to understand how such things can possibly happen.
This phenomenon captured the imagination of mathematician and code-breaker Alan Turing, who famously described one mechanism for

the spontaneous emergence of periodic patterns. In a “Turing mechanism” the initially uniform secretion of a diffusible ‘activator’ molecule triggers the production of a faster-diffusing ‘inhibitor’. Over time, instabilities in the system become amplified until these molecules resolve into patterns. Examples of such patterns might include the stripes of a tiger or the spots of a leopard. Even non-leopards such as you and I have a spotty distribution of hair follicles in our skin, following a pattern that could be explained by a Turing mechanism.
In the paper that I’ve chosen to highlight for the PLOS Biology XV Collection, Glover et al [1] set out initially to identify the components of the putative Turing mechanism that patterns hair follicles. The authors used a beautiful live explant system that allowed them to follow patterning in real time. They successfully homed in on a signalling network that seemed to be sufficient to explain the distribution of hair follicles. They then observed that the first ‘pre-pattern’ emerges within the epidermal layer of the skin, and this then dictates the position of mesenchymal condensates—groupings of mesenchymal cells necessary for the formation of a new hair follicle—in the underlying dermis.
So far, so good: the pattern is explained. Now comes the surprise. Because the authors now knew which particular signals drive Turing patterning, they were able to disrupt the distribution of these signals and show that this wipes out the epidermal prepattern. Unexpectedly, a suitably spotty distribution of condensates still somehow emerged in the dermis. At first this looked very similar to the usual Turing pattern, but Glover et al noticed a few tell-tale differences. Instead of lining up neatly along cut edges of explants, spots now seem to avoid sitting too close to these edges. The dynamics of patterning was also altered. These careful observations combined with modelling suggested that this epidermis-independent spottiness was driven not by Turing patterning (which you’ll remember is driven by diffusion of activator and inhibitor molecules) but by a conceptually similar but mechanistically distinct mode where it is cells rather than molecules that move. The authors found that patterning is driven through local aggregation of cells. This clustering becomes reinforced locally as cells draw closer to each other and is inhibited more distantly because cells become sparser as they move away from the future interfollicular regions and towards the aggregates. Glover et al went on to identify the molecules that mediate this previously-cryptic mesenchymal self-organisation process and to reveal how it is linked with epidermal Turing patterning in a hierarchical process.
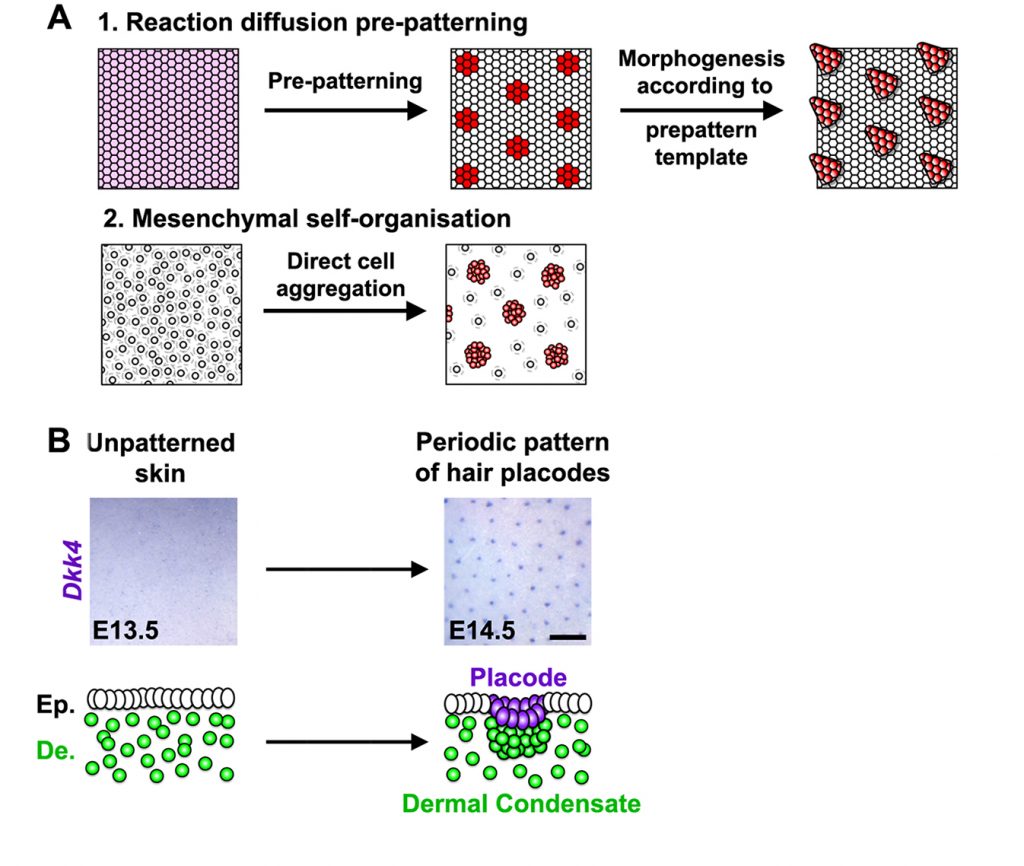
These findings have implications beyond explaining our hairiness. They make us look with a fresh eye at other patterning processes and wonder whether there may be additional cryptic mechanisms lurking undiscovered behind our textbook models. For example, it has long been known that patterning at gastrulation is dictated in mammals by signalling centres in the extraembryonic tissues, so it came as a shock when it was recently discovered that similar patterns somehow still emerge within aggregates of pluripotent cells even in the absence of extraembryonic tissues [2-5]. Are there two distinct but interlinked mechanisms operating at gastrulation, just as there are in the skin?
The study raises a number of other questions. Is the cryptic “secondary” patterning mechanism merely a remnant of evolutionary history, or could it be important for ensuring that patterning is robust? Could the principle of using multiple interacting patterning mechanisms be useful in guiding efforts to engineer patterns into groups of cells [6]? Perhaps the broadest lesson here is that we should not rush to decide between apparently competing hypotheses on the assumption that one of them must be wrong. Sometimes biology really does let us have it both ways.
References
1. Glover JD, Wells KL, Matthäus F, Painter KJ, Ho W, Riddell J, et al. Hierarchical patterning modes orchestrate hair follicle morphogenesis. PLoS biology. Public Library of Science; 2017;15: e2002117. doi:10.1371/journal.pbio.2002117
2. Berge ten D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3: 508–518. doi:10.1016/j.stem.2008.09.013
3. Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods. 2014;11: 847–854. doi:10.1038/nmeth.3016
4. Turner DA, Girgin M, Alonso-Crisostomo L, Trivedi V, Baillie-Johnson P, Glodowski CR, et al. Anteroposterior polarity and elongation in the absence of extraembryonic tissues and spatially localised signalling in Gastruloids, mammalian embryonic organoids. Development (Cambridge, England). 2017. doi:10.1242/dev.150391
5. Blin G, Wisniewski D, Picart C, Théry M, Puceat M, Lowell S. Geometrical confinement controls the asymmetric patterning of brachyury in cultures of pluripotent cells. Development (Cambridge, England). Oxford University Press for The Company of Biologists Limited; 2018;145: dev166025. doi:10.1242/dev.166025
6. Davies J. Using synthetic biology to explore principles of development. Development (Cambridge, England). 2017;144: 1146–1158. doi:10.1242/dev.144196
Sally Lowell is a Wellcome Trust Senior Research Fellow at the MRC Centre for Regenerative Medicine at the University of Edinburgh, and is a member of the PLOS Biology Editorial Board.
This blog post is the eleventh in a series of twelve, forming PLOS Biology’s XV Collection, celebrating 15 years of outstanding open science; read Lauren Richardson’s blog for more information.
Featured image credit: Flickr user Amy Chiang
Sally Lowell image credit: Wellcome Library, London. Wellcome Images

